Training
Does your company need specialized training of your key personnel and/or of company teams?
GxP-Pharma Support A/S organizes training in relevant topics within several GxP regulatory requirements. Our company has experience in all types GMDP-production, laboratories, QA/QC, GDP areas, several ISO standards, pharmacovigilance and The Blood Act. You can read more by selecting the GMP menu; the professional expertise.
We organize exactly the type of training, your business needs, taking into account your internal requirements and applicable regulatory requirements.
Training can easily be adapted to the individual groups of staff, to ensure the training will be more relevant for the specific employees. You can choose whether GxP-Pharma Support A/S shall train your staff on-site or in our suitable premises in Soro. We have different conference rooms with AV equipment and upon request we offer catering during the course.
GxP-Pharma Support A/S adjusts the courses as half-day or full-day training courses according to your company needs. The training can also be established on hourly basis.
Inspiration to specific courses
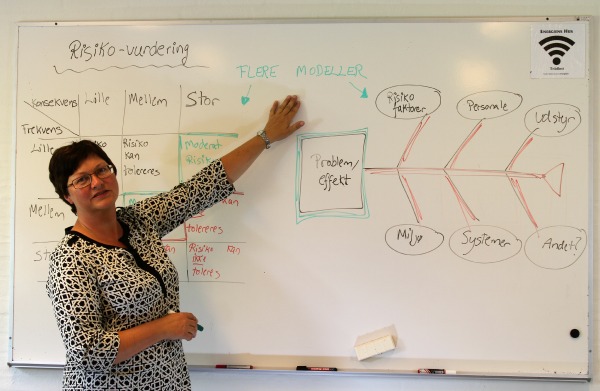
Does your company needs support to establish risk management courses at a specific level and according to the complexity of your company, GxP-Pharma Support A/S provides company-specific courses adjusted to relevant focus areas, either carried out at your location or at our premises in Soro.
GxP-Pharma Support A/S also provides general training in the new GDP requirements. This training can be performed on-site at your location or at our premises.
We also focus at specialized GMP and GDP training of new recruits in production or warehouses; this includes registration and documentation for the training as well, This can be organized according to the employee tasks, based on the daily work within their functional areas. The training can take place on-site in the warehouse, the packing area etc., close to the working environment.